Integrum’s OPRA™ Implant System now offered at a leading academic medical center in North Carolina
The program will offer comprehensive, highly specialized care for patients who have lost limbs due to trauma or cancer.
Duke Health and Duke University Hospital will be offering osseointegration using Integrum’s OPRA™ Implant System. Duke University Hospital, an award-winning hospital, as recognized by U.S. News and World Report, will be one of few leading institutions in the country to offer this highly specialized, comprehensive care program. This program is made available at Duke through an agreement between Integrum and Onkos Surgical.
Brian Brigman, MD, PhD, an orthopaedic surgical oncologist with the Department of Orthopaedics, is leading this multidisciplinary osseointegration program. “The team at Duke is committed to delivering better quality of life and better outcomes for our amputee patients,” says Brigman.
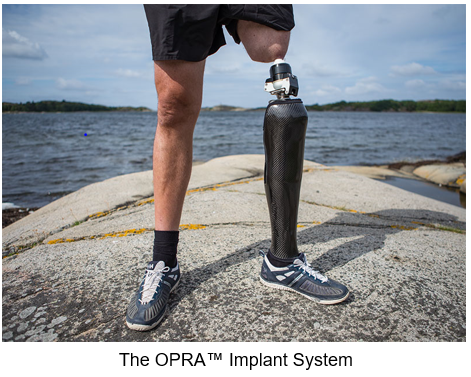
Approximately 2 million people in the U.S. live with limb loss. Up to three quarters of patients who undergo a lower-extremity amputation experience post-surgical issues in the prosthetic socket. As an alternative to conventional socket prostheses, bone-anchored prostheses allow direct connection of an artificial limb to the skeleton. The technology, commonly referred to as osseointegration, improves control over the prosthetic limb, increases joint and limb mobility, reduces pain, and eliminates the risk of further complications associated with a socket. This improvement in function and mobility affords amputees a new opportunity to return to a normal, active life.
Maria Lopez, CEO of Integrum shared “Duke Health has a wonderful track record in providing leading personalized patient care, and the formation of this team is a great step further on that mission.”
To make an appointment with a Duke orthopaedic surgeon or learn more about its services, visit https://www.dukehealth.org/treatments/orthopaedics or call (919) 980-5785.
About the OPRA™ Implant System
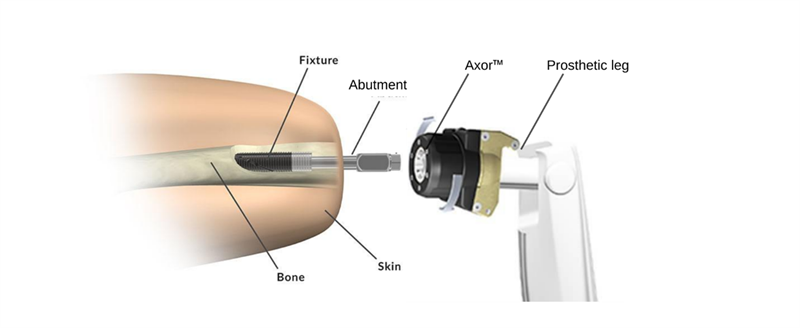
Integrum’s OPRA™ Implant System is the only FDA-approved, bone-anchored prosthetic solution available in the United States. OPRA™ Implant System consists of an anchorage element (Fixture) and a skin penetrating device (Abutment). The Fixture is surgically inserted into the femur, and after a healing time of several months, the Abutment is connected to the Fixture. The prosthetic leg is then attached directly to the Abutment via the Axor™, a prosthetic connection and safety device.
This disclosure contains information that Integrum AB is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 04-11-2020 08:30 CET. Integrum is listed on Nasdaq First North in Stockholm. Erik Penser is the Company's Certified Adviser.
For further information, please contact:
Maria Lopez, CEO
Cell. +46 (0) 708-46 10 69
Email: maria.lopez@integrum.se
Certified Adviser:
Erik Penser Bank AB
Tel. +46 (0) 8 463 8000
Email: certifiedadviser@penser.se
